- 0391-6109928
- 15302105619
- export01@zw-pvp.com
- Language:

Appearance:clear liquid
Purity:99%
Jiaozuo Zhongwei Special Products Pharmaceutical Co., Ltd. (hereinafter referred to as Zhongwei) is a manufacturer specializing in the production of pharmaceutical excipients and a national high-tech enterprise. Our innovative cooperation with domestic universities and continuous R&D investment ensure that the quality of our products is always at the international level, and hence we can continue to provide customers with better quality products. The company's quality management system adheres to the PDCA principle, focusing on customer needs and meeting their requirements for product quality. It follows ICH, cGMP, and EXCiPACT standards, integrating quality risk management throughout the entire product lifecycle. This includes supplier audits and approvals, procurement of raw materials and auxiliary materials, production process control, change control, and deviation management.
Chemical Properties
clear liquid
Uses
1-Ethyl-2-pyrrolidone was used to modify coir fiber (from Cocos nucifera) by photocuring.
General Description
1-Ethyl-2-pyrrolidone is a transdermal absorption-enhancing compound and mechanism of its effect on multilammellar liposome of stratum corneum lipid has been studied.
Flammability and Explosibility
Nonflammable
Safety Profile
Moderately toxic by ingestion. An eye irritant. Combustible. When heated to decomposition it emits toxic fumes of NOx.
InChI:InChI=1/C6H11NO/c1-2-7-5-3-4-6(7)8/h2-5H2,1H3
-
-
-
Rapid analysis of single and scant cell populations is essential in modern diagnostics, yet existing methods are often limited and slow. Herein, we describe an ultra-fast, highly efficient cycling method for the analysis of single cells based on unique li
The selective hydrogenation of functionalized olefins is of great importance in the chemical and pharmaceutical industry. Here, we report on a nanostructured nickel catalyst that enables the selective hydrogenation of purely aliphatic and functionalized olefins under mild conditions. The earth-abundant metal catalyst allows the selective hydrogenation of sterically protected olefins and further tolerates functional groups such as carbonyls, esters, ethers and nitriles. The characterization of our catalyst revealed the formation of surface oxidized metallic nickel nanoparticles stabilized by a N-doped carbon layer on the active carbon support.
Booming of photocatalytic water splitting technology (PWST) opens a new avenue for the sustainable synthesis of high-value-added hydrogenated and oxidized fine chemicals, in which the design of efficient semiconductors for the in-situ and synergistic utilization of photogenerated redox centers are key roles. Herein, a porous polymeric carbon nitride (PPCN) with a crystalline backbone was constructed for visible light-induced photocatalytic hydrogen generation by photoexcited electrons, followed by in-situ utilization for olefin hydrogenation. Simultaneously, various alcohols were selectively transformed to valuable aldehydes or ketones by photoexcited holes. The porosity of PPCN provided it with a large surface area and a short transfer path for photogenerated carriers from the bulk to the surface, and the crystalline structure facilitated photogenerated charge transfer and separation, thus enhancing the overall photocatalytic performance. High reactivity and selectivity, good functionality tolerance, and broad reaction scope were achieved by this concerted photocatalysis system. The results contribute to the development of highly efficient semiconductor photocatalysts and synergistic redox reaction systems based on PWST for high-value-added fine chemical production.
The reaction of N-trimethylsilyl derivatives of amides and imides with alkyl sulfonates on heating affords the corresponding N-alkyl derivatives and trimethylsilyl sulfonates.
Herein, we report a series of easily accessible bidentate N-heterocyclic carbene (NHC) cobalt catalysts, which enable the hydrogenation of hindered alkenes under mild conditions. The four-coordinated bidentate NHC-Co(II) complexes were characterized by X-ray diffraction, elemental analysis, ESI-HRMS, and magnetic moment measurements, revealing a distorted-tetrahedral geometry and a high-spin configuration of the metal center. The activity of the in situ formed catalytic system, which was obtained from easily available NHC precursors, CoCl2, and NaHBEt3, was identical with those of well-defined NHC-cobalt catalysts. This highlights the potential utility of this reaction system.
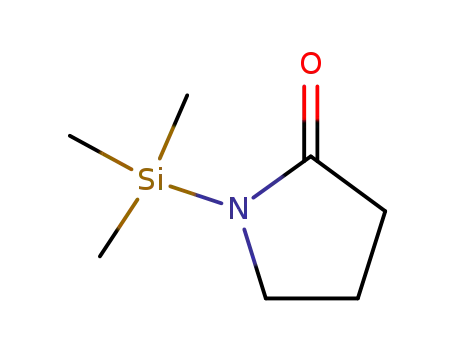
N-trimethylsilyl-pyrrolidin-2-one

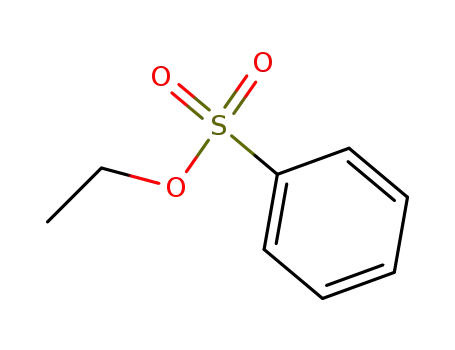
Ethyl benzenesulfonate

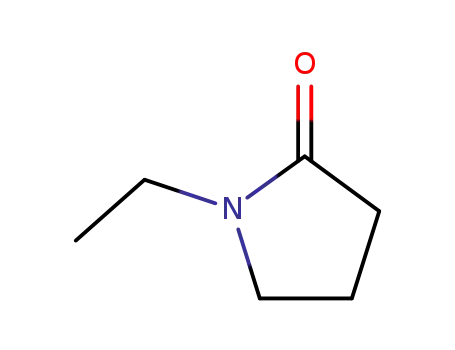
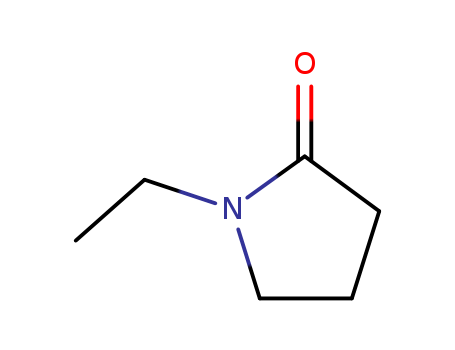
1-ethyl-2-pyrrolidinone

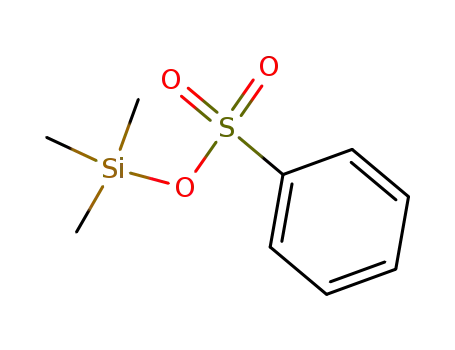
trimethylsilyl benzenesulfonate
| Conditions | Yield |
|---|---|
|
Heating;
|
59% 72% |
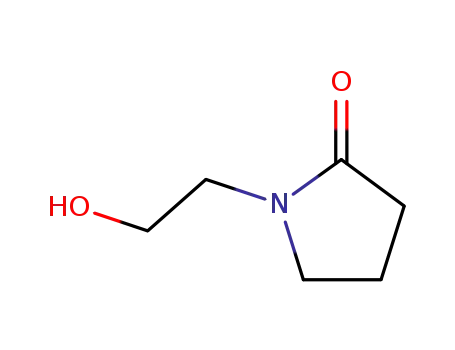
1-(2-hydroxyethyl)-2-pyrrolidinone

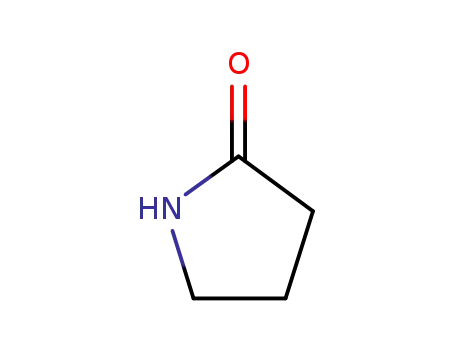
2-pyrrolidinon


1-ethyl-2-pyrrolidinone

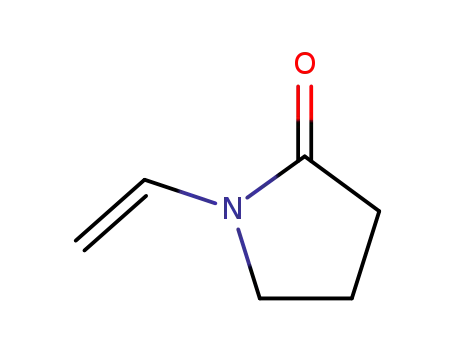
1-ethenyl-2-pyrrolidinone
| Conditions | Yield |
|---|---|
|
0.37 - 1.6 % cesium on silica; In water; at 350 - 375 ℃; for 3 - 95h; Product distribution / selectivity;
|
|
|
0.37 - 1.6 % cesium on silica; at 350 - 375 ℃; for 3 - 95h; Product distribution / selectivity;
|
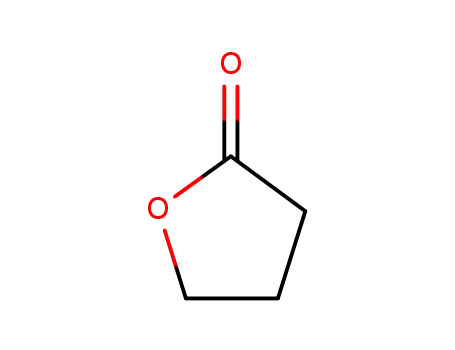
4-butanolide
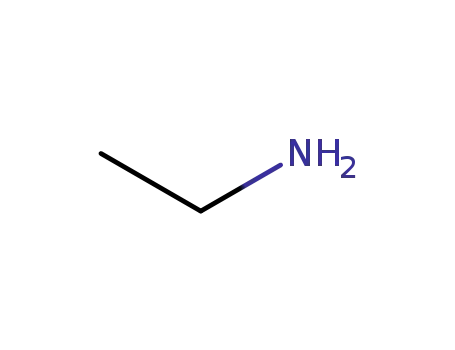
ethylamine
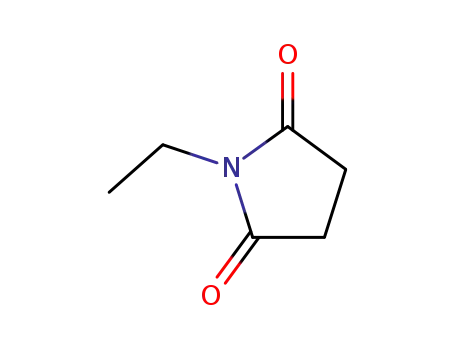
N-ethylsuccinimide
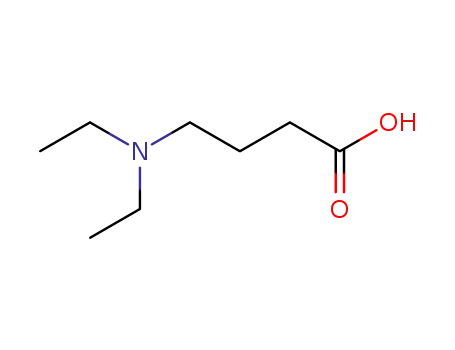
4-(N,N-diethylamino)butanoic acid
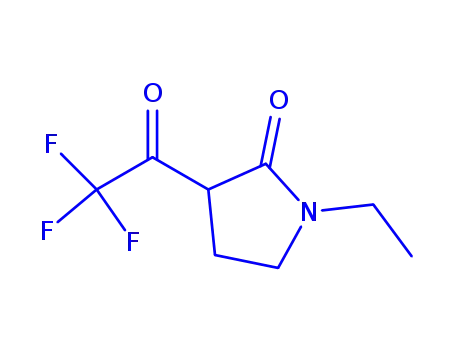
N-ethyl-3-trifluoroacetyl-2-pyrrolidone
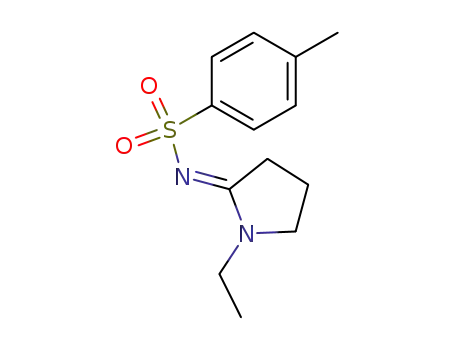
(E)-N-(1-ethylpyrrolidin-2-ylidene)-4-methylbenzenesulfonamide

N-ethylsuccinimide
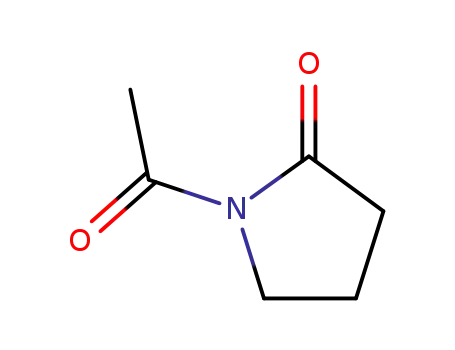
N-acetylpyrrolidinone