- 0391-6109928
- 15302105619
- export01@zw-pvp.com
- Language:

Appearance:clear colorless liquid or low melting solid
Purity:99%
Zhongwei has always adhered to the corporate purpose of "winning trust with sincerity and quality" and established a corporate image of "high technology, high standards and high requirements"; depending on reliable quality assurance, advanced management experience and scientific market analysis, its products have been exported to many countries and regions such as Europe, America, Southeast Asia and the Middle East. Our company strictly follows the corporate philosophy of "Quality Is the Backbone of an Enterprise", follows cGMP regulations, and has established a strict quality management system (QMS), environmental and occupational health and safety system (EHS). The company's quality management system adheres to the PDCA principle, focusing on customer needs and meeting their requirements for product quality. It follows ICH, cGMP, and EXCiPACT standards, integrating quality risk management throughout the entire product lifecycle. This includes supplier audits and approvals, procurement of raw materials and auxiliary materials, production process control, change control, and deviation management. All requirements for quality control are systematically implemented throughout the product development, production, control, release, storage, and shipping processes.
|
616-45-5 Name |
|
|
Name |
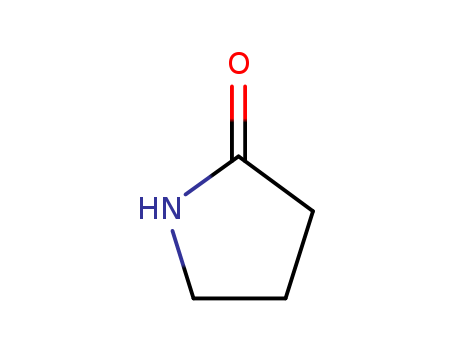
2-Pyrrolidinone |
|
Synonym |
2-Pyrrolidone Butyrolactam;2-AZACYCLOPENTANONE;2-P;2-KETOPYRROLIDINE;ALPHA-PYRROLIDONE;PIPERIDINIC ACID LACTAM;2-Oxopyrrolidine;2-Pyrol |
|
616-45-5 Biological Activity |
|
|
Description |
2-Pyrrolidinone is an active compound present in Brassica oleracea var. capitata and displays anticancer property. |
|
Related Catalog |
Research Areas >> Cancer |
|
Target |
Human Endogenous Metabolite |
|
In Vitro |
Radical scavenging activity increases with increasing the amount of 2-Pyrrolidinone. The cytotoxicity of 2-Pyrrolidinone is found to be dose and time dependent, and the effect is observed microscopically in all of the selected cancer cell lines. It is observed that 2-Pyrrolidinone has cytotoxic concentrations of 2.5 mg/mL for HeLa, 3 mg/mL for PC-3 cells at 24 h and 1.5 mg/mL for HeLa and 2 mg/mL for PC-3 cells at 48 h, respectively. The cell viability decreases with increasing concentrations of purified 2-Pyrrolidinone. After treatment with 2-Pyrrolidinone their IC50 concentrations (1.5 mg/mL and 2 mg/mL for HeLa and PC-3 cells, respectively) for a period of 24 h, morphological changes in the cells are observed. It is demonstrated that 2-Pyrrolidinone induces apoptosis in HeLa and PC-3 cells via G0/G1phase of cell cycle arrest[1]. |
|
Cell Assay |
The inhibitory concentrations (IC50) are evaluated using an MTT assay. Cancer cells are grown (1×104 cells/well) in a 96-well plate for 48 h into 75% confluence. The medium is replaced with fresh medium containing concentration of (0.5 to 5 mg/mL) 2-Pyrrolidinone, and the cells are further incubated for 24 h and 48 h. The culture medium is removed, and 100 mL of the MTT solution is added to each well and incubated at 37°C for 4 h. After removal of the supernatant, 50 mL of DMSO is added to each of the wells and incubated for 10 min to solubilize the formazan crystals. The optical density is measured at 620 nm in an ELISA multi-well plate reader. The OD value is used to calculate the percentage of viability[1]. |
|
References |
[1]. Thangam R, et al. Antioxidant and in vitro anticancer effect of 2-pyrrolidinone rich fraction of Brassica oleracea var. capitata through induction of apoptosis in human cancer cells. Phytother Res. 2013 Nov;27(11):1664-70. |
|
616-45-5 Chemical & Physical Properties |
|
|
Melting point |
25.5 ºC |
|
Boiling point |
250 ºC |
|
Density |
1.103 |
|
Molecular Formula |
C4H7NO |
|
Molecular Weight |
85.104 |
|
Flash Point |
138 ºC |
|
PSA |
29.10000 |
|
LogP |
-1.01 |
|
Exact Mass |
85.052765 |
|
Vapour density |
2.9 (vs air) |
|
Vapour Pressure |
0.1±0.8 mmHg at 25°C |
|
Index of Refraction |
1.486-1.488 |
|
Storage condition |
2-8°C |
|
Water Solubility |
miscible |
|
616-45-5 Description |
|
2-Pyrrolidinone occurs as a colorless or slightly grayish liquid, as white or almost white crystals, or colorless crystal needles. It has a characteristic odor. miscible with water, alcohol, ether, chloroform, benzene, ethyl acetate and carbon disulfide, insoluble in petroleum ether. |
|
616-45-5 Uses |
|
|
616-45-5 Production Methods |
|
The synthesis of 2-pyrrolidone was first reported in 1889 as the product of dehydration of 4-aminobutanoic acid. It is produced commercially by condensation of butyrolactone with ammonia, a method first described in 1936. Other synthetic routes include carbon monoxide insertion into allylamine, hydrolytic hydrogenation of succinonitrile, and hydrogenation of ammoniacal solutions of maleic and succinnic acids (Hort and Anderson 1978). |
|
616-45-5 Safety |
|
Pyrrolidones are mainly used in veterinary injections and have also been suggested for use in human oral, topical, and parenteral pharmaceutical formulations. In mammalian species, pyrrolidones are biotransformed to polar metabolites that are excreted via the urine. Pyrrolidone is mildly toxic by ingestion and subcutaneous routes; mutagenicity data have been reported. LD50 (guinea pig, oral): 6.5 g/kg LD50 (rat, oral): 6.5 g/kg |