- 0391-6109928
- 15302105619
- export01@zw-pvp.com
- Language:

Appearance:liquid
Purity:99%
Jiaozuo Zhongwei Special Products Pharmaceutical Co., Ltd. (hereinafter referred to as Zhongwei) is a manufacturer specializing in the production of pharmaceutical excipients and a national high-tech enterprise. The company has an excellent technical, marketing and management team, equipped with a complete set of production and testing equipment; focusing on the research and development, production and sales of polyvinylpyrrolidone (PVP) series products, and is committed to providing high-quality polyvinylpyrrolidone products and solutions to global customers. The company's quality management system adheres to the PDCA principle, focusing on customer needs and meeting their requirements for product quality. It follows ICH, cGMP, and EXCiPACT standards, integrating quality risk management throughout the entire product lifecycle. This includes supplier audits and approvals, procurement of raw materials and auxiliary materials, production process control, change control, and deviation management. All requirements for quality control are systematically implemented throughout the product development, production, control, release, storage, and shipping processes.
|
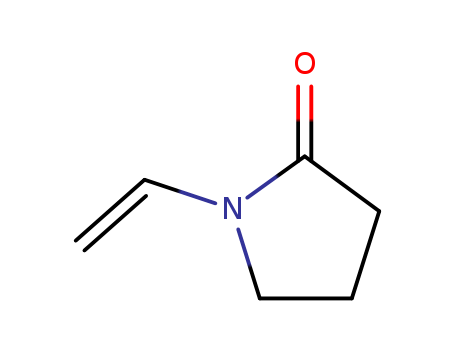
88-12-0 Name |
|
|
Name |
N-VINYLPYRROLIDONE |
|
Synonym |
N-Vinyl-2-pyrrolidone;1-VINYL-2-PYRROLIDINONE, 99+%;N-Vinyl-2-pyrrolidone, stabilized with Kerobit;N-Vinyl-2-pyrrolidone, stabilized, 98%;N-VINYL-2-PYRROLIDINONE;N-VINYL-2-PYRROLIDONE;N-VINYLPYRROLIDONE;N-VINYLBUTYROLACTAM;1-ethenyl-2-pyrrolidinon |
|
88-12-0 Chemical & Physical Properties |
|
|
Melting point |
13-14 °C |
|
Boiling point |
249.1±29.0 °C at 760 mmHg |
|
Density |
1.1±0.1 g/cm3 |
|
Molecular Formula |
C6H9NO |
|
Molecular Weight |
111.142 |
|
Flash Point |
134.7±9.2 °C |
|
PSA |
20.31000 |
|
LogP |
-0.72 |
|
Exact Mass |
111.068413 |
|
Vapour density |
3.8 (vs air) |
|
Vapour Pressure |
0.0±0.5 mmHg at 25°C |
|
Index of Refraction |
1.554 |
|
Storage condition |
0-6°C |
|
Stability |
Stable. Incompatible with strong oxidizing agents. |
|
Water Solubility |
miscible |
|
88-12-0 Description |
|
N-vinyl-2-pyrrolidone (NVP) is commonly used as a reactive diluent for radiation curing in UV-coating, UV-inks, and UV adhesives. It is used as a monomer to produce water soluble polyvinylpyrrolidone (PVP) with uses in pharmaceuticals, oil field, cosmetics, food additives & adhesives. It is used in the manufacture of copolymers with, for example, acrylic acid, acrylates, vinyl acetate and acrylonitrile and in the synthesis of phenolic resins. 1-Vinyl-2-pyrrolidinone is a colorless to yellow liquid, with a characteristic odor. Its melting point is around 13.5oC and boils at about 90-92oC. VP is completely miscible in water and in most organic solvents but partially miscible in aliphatic hydrocarbons. Industrial production of VP by reacting 2-pyrrolidone with acetylene at high pressure and temperature has been reported. The vinylation process proceeds in liquid phase and is catalyzed by 3-pyrrolidone -potassium hydroxide. |
|
88-12-0 Uses |
|
|
88-12-0 Safety Profile |
|
88-12-0 Safety ProfileConfirmed carcinogen. Moderately toxic by ingestion, inhalation, and skin contact. A severe eye irritant. Probably irritating and narcotic in high concentrations. Combustible when exposed to heat or flame; can react vigorously with oxibzing materials. To fight fire, use alcohol foam, CO2, dry chemical. When heated to decomposition it emits highly toxic fumes of NOx. |